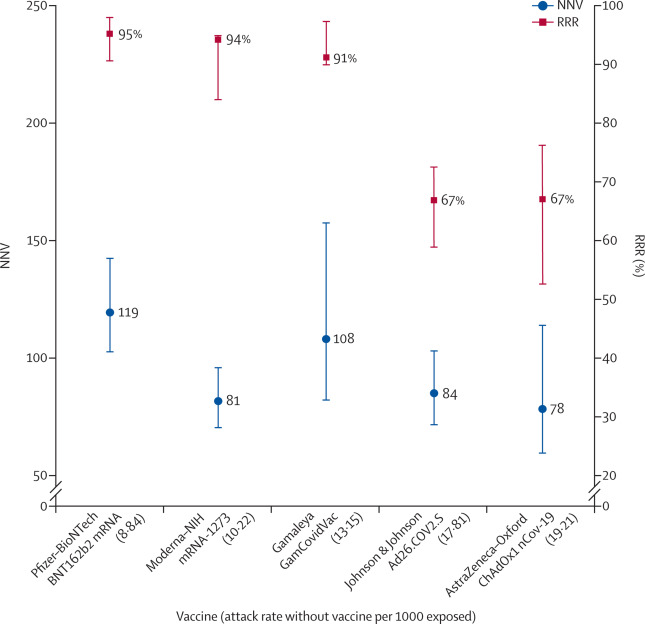
both the numerator and denominator change, RRR does not change (66–67%), but the one-third increase in attack rates in the unvaccinated group (from 1·8% to 2·4%) translates in a one-fourth decrease in NNV (from 84 to 64).
When communicating about vaccine efficacy, especially for public health decisions such as choosing the type of vaccines to purchase and deploy, having a full picture of what the data actually show is important, and ensuring comparisons are based on the combined evidence that puts vaccine trial results in context and not just looking at one summary measure, is also important. Such decisions should be properly informed by detailed understanding of study results, requiring access to full datasets and independent scrutiny and analyses.
Dagan and colleagues
report an RRR of 94%, which is essentially the same as the RRR of the phase 3 trial (95%) but with an ARR of 0·46%, which translates into an NNV of 217 (when the ARR was 0·84% and the NNV was 119 in the phase 3 trial). This means in a real-life setting, 1·8 times more subjects might need to be vaccinated to prevent one more case of COVID-19 than predicted in the corresponding clinical trial.
Supplementary Material
-
Supplementary appendix
References
- 1.
Covid-19 Vaccine Tracker.https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.htmlDate accessed: March 10, 2021
- 2.
Safety and efficacy of the BNT162b2 mRNA COVID-19 Vaccine.
N Engl J Med. 2020; 383: 2603-2615
- 3.
Efficacy and safety of the mRNA-1273 SARS-CoV-2 Vaccine.
N Engl J Med. 2021; 384: 403-416
- 4.
Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK.
Lancet. 2021; 397: 99-111
- 5.
Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia.
Lancet. 2021; 397: 671-681
- 6.
Vaccines and Related Biological Products Advisory Committee meeting: FDA briefing document.https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-december-10-2020-meeting-announcementDate: Dec 10, 2020Date accessed: March 10, 2021
- 7.
Vaccines and Related Biological Products Advisory Committee meeting: FDA briefing document.https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-december-17-2020-meeting-announcementDate: Dec 17, 2020Date accessed: March 10, 2021
- 8.
Vaccines and Related Biological Products Advisory Committee meeting: FDA briefing document.https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-february-26-2021-meeting-announcementDate: Feb 26, 2021Date accessed: March 10, 2021
- 9.
What does 95% COVID-19 vaccine efficacy really mean?.
Lancet Infect Dis. 2021; (published online Feb 17.)
- 10.
Outcome reporting bias in COVID-19 mRNA vaccine clinical trials.
Medicina (Kaunas). 2021; 57: 199
- 11.
BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting.
N Engl J Med. 2021; (published online Feb 24.)
Article Info
Publication History
Identification
Copyright
User License
Creative Commons Attribution – NonCommercial – NoDerivs (CC BY-NC-ND 4.0) |
ScienceDirect
Source: COVID-19 vaccine efficacy and effectiveness—the elephant (not) in the room – The Lancet Microbe
Disclaimer
Some of the posts we share are controversial and we do not necessarily agree with them in the whole extend. Sometimes we agree with the content or part of it but we do not agree with the narration or language. Nevertheless we find them somehow interesting, valuable and/or informative or we share them, because we strongly believe in freedom of speech, free press and journalism. We strongly encourage you to have a critical approach to all the content, do your own research and analysis to build your own opinion.
We would be glad to have your feedback.


