
There is currently a raging discussion going on in German-speaking news and social media about whether the leaked final redactions of the minutes of the Robert Koch Institute’s Covid-19 crisis group are in fact forgeries. At least one now ostensibly unredacted and highly publicised passage in the documents strongly suggests that they are.
The passage in question was highlighted by Aya Velazquez, the journalist to whom the documents were provided, in the July 23rd press conference that she held with two other German Covid-response critics.
It reads as follows:
EMA and Pfizer are considering whether they might skip the phase III trials and go directly to broad use, if the regulators decide to do this, then it [the authorisation process] could go quicker than 12-18 months.
The prior bullet point in the minutes affirms that “normally” the authorisation process of a new vaccine is expected to take “12-18 months from the start of phase I.”
The passage clearly alludes to the authorisation process of what has come to be commonly known as the “Pfizer” Covid-19 vaccine, even though the actual developer and legal manufacturer is the German company BioNTech. The EMA is the European Medicines Agency.
But the problem with the passage is, above all, the date of the minutes in which it is included: April 15th, 2020. The passage is wildly anachronistic. There is no reason why Pfizer should have had any contact with the EMA on authorisation of the BioNTech-Pfizer vaccine so early.
Indeed, there is no reason why Pfizer would have had any official, direct contact even later. The applicant for the authorisation was not Pfizer. It was BioNTech. This is also the case for the application for full authorisation in the US. But in the US, BioNTech, as a foreign firm, had to appoint a domestic agent and it appointed Pfizer to play this role. The company had no need for any intermediary in the EU.
But in any case, on April 15th, 2020, neither company would have yet had the occasion to discuss their long-term plans with the EMA. BioNTech had only registered its Phase 1 trial of its Covid-19 vaccine candidate with the EMA literally just the day before! See the entry from the EMA’s Clinical Trials Register below.
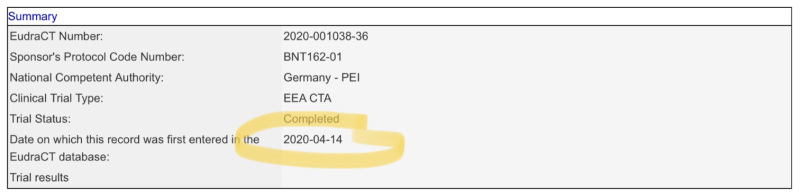
This registration will have been BioNTech’s first official contact with the EMA in the matter of its Covid-19 vaccine candidate. As indicated in the record, the trial was to be conducted under the aegis of the German regulatory agency, the Paul Ehrlich Institute (PEI).
At this stage, the national regulator, the PEI was undoubtedly BioNTech’s principal interlocutor. According to BioNTech CEO Ugur Sahin, he and his team first met with the PEI to discuss the company’s Covid-19 vaccine project more than two months earlier, on February 6th. (See The Vaccine, p.52.)
It is important to note that while BioNTech and Pfizer had concluded their Covid-19 vaccine collaboration agreement in mid-March, Pfizer was not involved in the German Phase I trial, which was registered with the EMA on April 14th and which began with the first vaccination of a human subject in Mannheim on April 23rd.
We do know that Sahin was in fact extremely concerned about accelerating the pace of the authorisation process. But it’s hard to imagine that in mid-April 2020 he was thinking so far ahead to the Phase III trials. Having not yet initiated the “first in human” Phase I trial, how could he even know he would get there?
But normal regulatory steps had to be skipped or somehow abridged for BioNTech even to be able to initiate its Phase I human trial in April. In particular, BioNTech had to be allowed to initiate the human trial without first completing a preclinical toxicology study on animals.
As discussed in detail here, the PEI in fact allowed BioNTech to do this, permitting the firm to start its human trial on the basis merely of an “interim” toxicology report before the final results were available. On Sahin’s account, BioNTech received the go-ahead from the PEI on April 21st. (See The Vaccine, p.173.)
It makes little sense for the RKI crisis team to have been discussing Pfizer and the EMA in the April 15th minutes. It would have made a lot of sense for them to be discussing BioNTech and the PEI. The RKI was surely being kept informed by its sister agency about the furious development of BioNTech’s vaccine project. (The two agencies bear roughly the same relationship as the CDC and the FDA in the United States.)
If not, Germany’s then Minister of Health Jens Spahn could have told RKI staff about it. In his April 1st, 2021 speech inaugurating BioNTech’s mRNA manufacturing facility in Marburg, Spahn said that he first met BioNTech CEO Sahin to discuss the company’s Covid-19 vaccine project “around 12 months ago.” This would take us to late March/early April.
The PEI is mentioned twice just a little further on in the same April 15th minutes, and the April 2nd minutes report that the PEI informed the RKI that a “clinical study will receive the authorisation tomorrow.” Curiously, the extant minutes do not tell us whose clinical study.
Other than in a November 11th quote from the British magazine Nature, Pfizer is never again mentioned in the 1,834 pages of the 2020 minutes independently of BioNTech. All further references are to “BioNTech-Pfizer,” “BioNTech/Pfizer,” and the like. BioNTech is often mentioned alone.
The complete RKI files in the versions provided to Aya Velazquez are available here. German observers quickly spotted that the leaked versions are not in fact identical to the official versions that had been previously released in redacted form to the journalist Paul Schreyer and then published, in already somewhat less redacted form, on the RKI website. (See here, for instance, for one comparison.) Whole passages from the previous release are in fact missing from the leaked version (and at least one of those passages, incidentally, contains redactions) and the language is not the same in others.
Velazquez explained the discrepancies by noting that she herself had received multiple versions of the documents from her source as Word docs and had selected those “closest” to the versions on the RKI website to include in the originally posted PDFs. She has, in the meanwhile, also posted these Word documents.
But, as critics have noted, a Word document can be altered at will. Some of the critics claim that metadata shows modifications being made – presumably by the Robert Koch Institute itself – well into 2024. In any case, what is the point of keeping minutes if they are going to be altered ex post? If the RKI created multiple versions of the crisis group minutes, what does it even mean to speak of an authentic version anymore?
Stop Press: Robert gave an interview to Jay Bhattacharya on his YouTube channel a couple of weeks ago that you can see here.
Republished from The Daily Sceptic
Disclaimer
Some of the posts we share are controversial and we do not necessarily agree with them in the whole extend. Sometimes we agree with the content or part of it but we do not agree with the narration or language. Nevertheless we find them somehow interesting, valuable and/or informative or we share them, because we strongly believe in freedom of speech, free press and journalism. We strongly encourage you to have a critical approach to all the content, do your own research and analysis to build your own opinion.
We would be glad to have your feedback.
Source: Brownstone Institute Read the original article here: https://brownstone.org/

