
Remember when there was panic in 2020 because Pfizer’s mRNA Covid-19 vaccine couldn’t be transported across the country unless it was stored at ultra-freezing temperatures?
Pfizer said the mRNA in the vaccine, which coded for the spike protein, was unstable and would decay if the unopened vials were not kept at -70ºC.
So, when the FDA granted authorisation in December 2020, it specified the vaccine had to be stored between -80ºC and -60ºC, requiring special ultra-cold freezers, which proved challenging to areas with limited resources.
But by February 2021, Pfizer had apparently solved the problem.
It submitted new “RNA stability data” to the FDA demonstrating the vaccine could be stored in conventional freezers (-20ºC) and no longer required ultra-cold freezers.
The FDA approved the change swiftly.
Two months later, Australia’s Therapeutic Goods Administration (TGA) also approved Pfizer’s application, allowing unopened vials to be stored at -20ºC for up to 2 weeks.
Storage temperature wasn’t the only change. Drug regulators also approved extensions to the vaccines’ expiry dates.
Various batches of Pfizer’s vaccine, for example, had their expiry dates extended by one year (FDA) or 6 months (TGA).
But given the sensitivity of RNA to changes in temperature and storage duration, what stability data did the regulators rely on to green-light these decisions?
Hitting a Brick Wall
I asked the FDA for the “RNA stability data” it received from Pfizer, but the agency said it would not provide the information.
Instead, the FDA instructed me to submit a Freedom of Information (FOI) request.
I complained to the FDA that its FOI process had stagnated and that I’d already submitted an FOI over 6 months ago which was still being “processed,” but to no avail.
Similarly, I asked the TGA for the data, but the agency said, “TGA is unable to provide this information directly as it is considered to be commercial in confidence by the Sponsors.”
And what about Pfizer? I received the same response. The company refused to disclose the data, saying it was “commercial in confidence.”
Phillip Altman has over 40 years’ experience in clinical trials and regulatory affairs, and says data on RNA stability is of huge public interest and should be disclosed.

“It’s critically important to know about the stability of RNA in the vaccines because if the RNA disintegrates, then the efficacy of the vaccine goes down,” says Altman.
“But my concern is more about safety because some people will receive higher doses of mRNA than others, and this might explain why some batches of the vaccine are associated with more adverse events than others,” he adds.
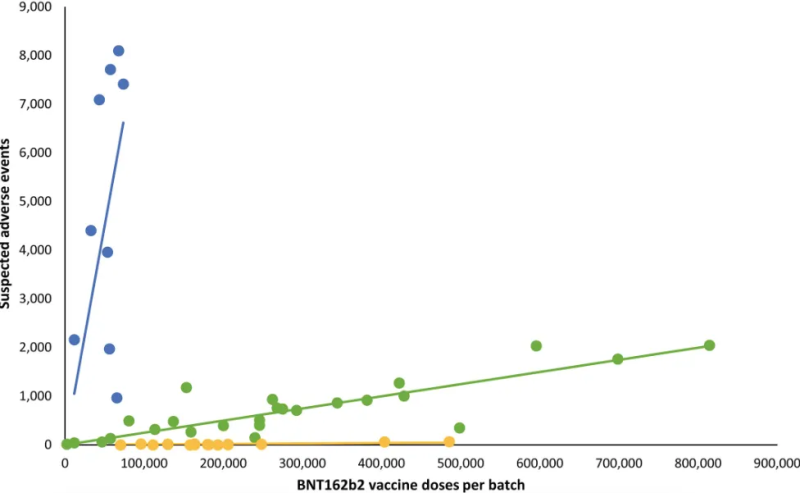
Altman points to a Danish analysis published in the European Journal of Clinical Investigation that found serious adverse events were strongly associated with particular batches of Pfizer’s Covid-19 vaccine (see graph).
David Wiseman, a PhD research bioscientist involved in medical product development says it’s not just “intact RNA” that we should be concerned about.
“We need to know about the bits of RNA that are not intact,” says Wiseman. “It’s possible that small fragments of mRNA also have biological effects such as inflammation or controlling how other RNA works.”

Wiseman says it’s not the first time regulators made a decision that could impact RNA stability, referring to the FDA’s approval to allow a change in the buffer solution used in Pfizer’s mRNA vaccine, claiming it “improved the stability profile of the vaccine.”
“If the new buffer helped stabilise the mRNA, then it would probably impact the amount of spike protein being produced or alter the way the lipid nanoparticles behaved in the body. But where were the data when the FDA made that decision? The FDA never insisted the new formulation be tested, at least in animals, before it was injected into children,” says Wiseman.
He pointed this out to the CDC in October 2021. Since then, Moderna has published research demonstrating how the change in buffer not only alters the way the mRNA works, but how it influences RNA stability.
Given the known stability problems, Wiseman says it would’ve been essential to conduct stability tests in real-world conditions to assess the integrity of the RNA and lipid nanoparticles after transportation, storage, and preparation and holding in clinics under non-ideal conditions.
“It’s time for regulators to restore public trust and release these sorts of data. Until then, why should we inject anyone, especially children, with a vaccine without disclosing these, and other kinds of data?” says Wiseman.
Republished from the author’s Substack
Disclaimer
Some of the posts we share are controversial and we do not necessarily agree with them in the whole extend. Sometimes we agree with the content or part of it but we do not agree with the narration or language. Nevertheless we find them somehow interesting, valuable and/or informative or we share them, because we strongly believe in freedom of speech, free press and journalism. We strongly encourage you to have a critical approach to all the content, do your own research and analysis to build your own opinion.
We would be glad to have your feedback.
Source: Brownstone Institute Read the original article here: https://brownstone.org/

