Mpox, Project Bioshield: Everyone’s Dark Winter?
by Sonia Elijah at Brownstone Institute

In 2022, mpox (formerly known as monkey pox) drew worldwide attention, when more than 20 countries reported infections to the World Health Organization by May of that year. It led to WHO Director-General, Tedros Adhanom Ghebreyesus, declaring the mpox outbreak involving clade IIb of the virus in the Democratic Republic of the Congo and its expansion to neighbouring countries, a global health emergency.
The monkeypox virus is part of the same family of viruses (orthopoxviruses) as smallpox but is less severe. In 1980, the World Health Assembly announced that smallpox had been eradicated and recommended that all countries cease vaccination. Although labs in two countries still officially store smallpox samples (US and Russia).
Mpox was first identified in Denmark, in 1958, when two outbreaks of a pox-like disease occurred in lab monkeys, which is how it got its name – although monkeys are not considered to be reservoirs for the virus. The source of the virus is said to be unknown.
In 1970, the first human case of mpox was recorded in the Democratic Republic of the Congo. This was during a time when smallpox was being eradicated. The disease is considered to be endemic to countries in central and west Africa. The outbreaks have been concentrated among men who have sex with men.
In late September 2023, the emergence of a new variant of the virus was detected in central Africa. As of August 2024, more than 21,000 cases have been reported, with over 600 fatalities, nearly all in the Democratic Republic of the Congo.
On 14 August 2024, the World Health Organization declared the epidemic a public health emergency of international concern (PHEIC).
A month after the WHO’s announcement, the small Danish biotech firm, Bavarian Nordic, became the first in the world to receive approval for a monkeypox vaccine, JYNNEOS (known internationally as Imvamune or Imvanex), initially developed for smallpox but was rarely used.
In the wake of the September 2001 anthrax attacks, President George W. Bush signed into law the Project BioShield Act of 2004 (Project BioShield) “as part of a broader strategy to defend America against the threat of weapons of mass destruction. The purpose of Project BioShield is to accelerate the research, development, purchase, and availability of effective medical countermeasures against biological, chemical, radiological, and nuclear (CBRN) threats.”
Project Bioshield was a ten-year program managed by Biomedical Advanced Research and Development Authority (BARDA), part of the United States Department of Health and Human Services (HHS).
The act called for $5 billion for purchasing vaccines that would be used in the event of a bioterrorist attack, for civilian use. Since 2001, $50 billion has been allocated by the United States government to address the threat of biological weapons.
A key element of the Act allowed the stockpiling and distribution of vaccines that had not been tested for safety or efficacy in humans, “due to ethical concerns.” This is because the agents could not have been tested in humans without also exposing them to the threat – instead animal testing was used to establish efficacy.
Bavarian Nordic’s smallpox vaccine was developed under Project Bioshield. The US government partnered with it to develop Jynneos, primarily to prevent smallpox in the event of a bioterrorist attack. Another strategic, “public-private partnership,” which resulted in the creation of a billion-dollar shot was Moderna’s collaboration with the NIH’s Vaccine Research Centre. The Covid mRNA-based shot, Spikevax, became Moderna’s first (after ten years of failing to bring a drug or vaccine to market) and most lucrative product ever.
Bavarian Nordic president and CEO Paul Chaplin said:
Jynneos was also the first smallpox vaccine successfully developed under Project BioShield, a programme created by the US Congress in 2004 to accelerate the research, development, procurement, and availability of medical countermeasures against biological, chemical, radiological, and nuclear (CBRN) agents through public-private partnerships.
As mentioned earlier, Bavarian Nordic’s smallpox vaccine was rarely used but on the heels of the WHO announcing mpox as a PHEIC, orders started flooding in from around the world.
On September 18, Gavi, the Vaccine Alliance, “announced an advance purchase agreement (APA) to secure 500,000 doses of the MVA-BN® mpox vaccine (marketed as JYNNEOS® or IMVANEX®) to be supplied to countries in Africa impacted by the mpox outbreak.”
On a side note – in June 2001, just a few months before the anthrax attacks which led to Project Bioshield, a bioterrorism training exercise, code-named “Dark Winter” simulated a covert smallpox attack on the United States. It was a collaborative effort led by the Johns Hopkins Center for Civilian Biodefense Strategies, the ANSER Institute for Homeland Security, and the Oklahoma City National Memorial Institute for the Prevention Terrorism.
Those who were present included several Congressmen, a former CIA director (James Woolsey), Tara O’Toole (then Director of Johns Hopkins Center for Civilian Biodefense Strategies, now Director of the CIA hedge fund In-Q-Tel), and CIA’s former deputy director for Science and Technology (Ruth David) and selected members of the press.
Those who participated in “Dark Winter” explored strategies for imposing coercive quarantines; censorship; mandatory masking, lockdowns, and vaccination; and expanded police powers as the only rational responses to the pandemic. Eerily, these strategies were adopted as governmental Covid countermeasures, decades later.
The organizer of this pandemic simulation was former Air Force physician Robert Kadlec, who also happened to lead “Crimson Contagion” in 2019 – another pandemic simulation run by the Department of Health and Human Services (HHS) involving a scenario in which a group of tourists returning from China spread a novel influenza virus in the United States.
Robert Kadlec served as Assistant Secretary of Health and Human Services (Preparedness and Response) from 2017-2021. He was responsible for the creation of Operation Warp Speed, the Covid vaccine development program.
A second smallpox vaccine that was approved by the US Food and Drug Administration (FDA) for the prevention of mpox was ACAM2000, on August 29th, under the Expanded Access Investigational New Drug (EA-IND) protocol.
ACAM2000 is manufactured by the controversial, Emergent BioSolutions. It was originally approved to prevent smallpox in 2007.
The side effects of ACAM2000 make a horrifying read.
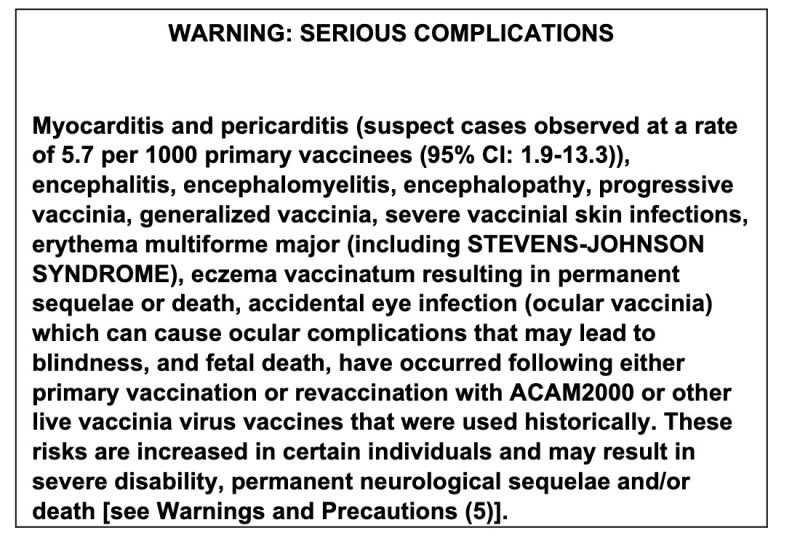
Not only is death listed as a “serious complication” but alarmingly the FDA’s own medication guide states: “ACAM2000 contains live vaccinia virus that can be transmitted to persons who have close contact with the vaccinee and the risks in contacts are the same as those for the vaccinee.”
Meaning that even people who come into close contact with someone who has been vaccinated could possibly die. The packaging insert even states this: “Death has also been reported in unvaccinated contacts accidentally infected by individuals who have been vaccinated.”
According to the CDC:
ACAM2000 is a second-generation vaccine that contains a live vaccinia virus that replicates efficiently in humans. It is manufactured by Emergent Bio Solutions and is indicated for the prevention of smallpox. It has been made available for use against mpox in the clade II outbreak under an Expanded Access Investigational New Drug (EA-IND) protocol, which requires informed consent along with completing additional forms…Although the United States has a large supply of ACAM2000, this vaccine has more side effects and contraindications than JYNNEOS.
Notably, on the vaccine information sheet for the other smallpox-turned-mpox vaccine, JYNNEOS, it reads: “CDC recommends consideration of the vaccine for people who administer ACAM2000®, or who care for patients infected with orthopoxviruses.”
In June of this year, Emergent BioSolutions “received more than $250 million in contract modifications from the Administration for Strategic Preparedness and Response (ASPR) at the United States Department of Health and Human Services (HHS), to deliver millions of doses of four medical countermeasures (MCMs)…These contract modifications will help ensure continued supply/stockpiling of critical MCMs to address biological threats and emergencies against anthrax, smallpox and botulism.”
One of the awards included a contract modification valued at $99.9 million to supply ACAM2000® this year.
What is interesting is that the FDA’s approval of ACAM2000 for the prevention of mpox came on the heels of Emergent BioSolutions pledge to donate 50,000 doses of its vaccine to “Democratic Republic of the Congo and other impacted countries of Burundi, Kenya, Rwanda and Uganda to address the current mpox outbreak.”
It is also noteworthy that this deadly vaccine was being stockpiled – with the CDC’s comment “the United States has a large supply,” nodding to that fact. The mpox outbreak provided the perfect opportunity to offload the surplus stock.
It is deeply disturbing that the FDA would approve a vaccine so life-alteringly harmful to not just those being given it – but to those who would come into close contact with the vaccinee. However, unlike the gene-based Covid shots that were branded as “vaccines,” at least the side effects are clearly stated on the medication guide for there to be some kind of informed consent.
This begs the question: how could the deadly ACAM2000 have gotten approval in the first place? Perhaps, “Dark Winter/ Crimson Contagion” Robert Kadlec and his connections might provide an answer.
Before being appointed by Trump as Assistant Secretary for Preparedness and Response in 2017, Kadlec was previously a consultant for none other than Emergent BioSolutions. The very same US biotechnology company that purchased ACAM2000 from Sanofi Pasteur in 2017. Kadlec was part-owner of a consulting company RPK Consulting providing services to Emergent, apparently until 2015. He chose not to disclose these facts in Senate nomination forms during his confirmation process in 2017.
Right after taking office, in a resounding conflict-of-interest move, Kadlec aggressively pushed to increase the government’s stockpile of smallpox vaccine through purchase agreements with Emergent BioSolutions. In the end, the HHS awarded a 10-year, $2.8 billion single-source contract to the company to purchase its smallpox vaccines at twice the previous price.
It is noteworthy that in 1998, Colonel Dr Robert Kadlec, who at the time was the Biodefence Programmes Director at the Department of Homeland Security, wrote in a Pentagon strategy paper: “Using biological weapons under the cover of an endemic or natural disease occurrence provides an attacker the potential for plausible denial. Biological warfare’s potential to create significant economic loss and subsequent political instability, coupled with plausible denial, exceeds the possibilities of any other human weapon.”
Republished from the author’s Substack
Mpox, Project Bioshield: Everyone’s Dark Winter?
by Sonia Elijah at Brownstone Institute – Daily Economics, Policy, Public Health, Society
Disclaimer
Some of the posts we share are controversial and we do not necessarily agree with them in the whole extend. Sometimes we agree with the content or part of it but we do not agree with the narration or language. Nevertheless we find them somehow interesting, valuable and/or informative or we share them, because we strongly believe in freedom of speech, free press and journalism. We strongly encourage you to have a critical approach to all the content, do your own research and analysis to build your own opinion.
We would be glad to have your feedback.
Source: Brownstone Institute Read the original article here: https://brownstone.org/

