
The worldwide spread of the coronavirus for weeks before it was first identified in the dying days of 2019 has continued to fascinate observers, not least because of what it implies for the futility of the extreme interventions based on the false belief that it was not already here.
This time last year I summarised what we then knew from studies and other evidence about this early spread. I argued that the evidence would suggest that the virus emerged around September or October 2019 in or near Wuhan and spread globally during that autumn and winter. However, it was not the dominant virus during that winter season and spread at low level without producing noticeable excess deaths (there was also none of the deadly medical, political, and social response at that time, of course).
Sadly, 2023 did not see much by way of additional evidence emerging on early spread. This is a pity as the World Health Organisation, back in 2020, rightly called on countries to investigate this critical matter in order to fill out our picture on the origin of the virus. It repeated this request in June 2022. However, during 2023 the WHO abandoned its origins investigation as hopeless due to a lack of cooperation from governments, meaning the prospect of getting to the bottom of this question is fading away.
One thing we can do, however, is attempt to glean new insights from the data we already have, and perhaps one day, when the current crop of suspiciously uninterested leaders has moved on, the trail will be picked up again by sincere people who want to get to the truth and have the resources to do it.
With this in mind, in recent days I have been looking again at some of the key early spread studies, in particular the one by Amendola et al. looking at molecular evidence in pre-pandemic samples from measles patients in Lombardy, Italy.
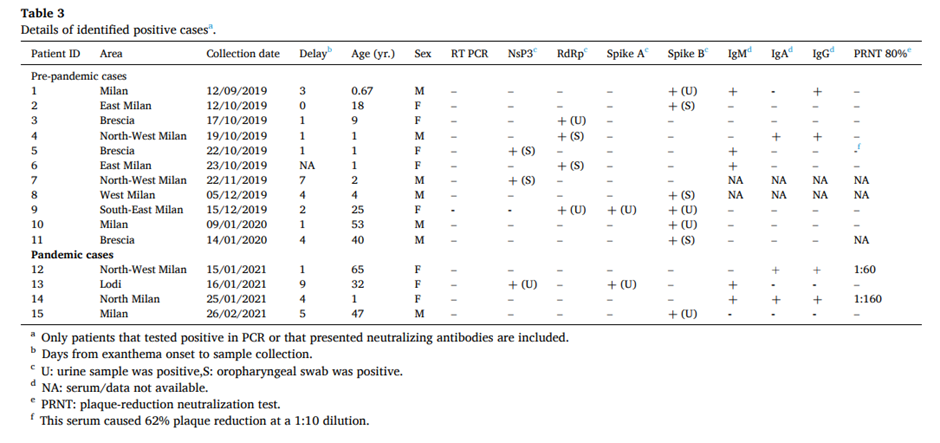
The main results are shown in the table above. After testing 44 samples from August 2019 to February 2020, 11 (25%) were positive for SARS-CoV-2 viral RNA. Note that this was not the complete viral genome. Instead the researchers tested only for certain fragments of the genome (note the table headings: NsP3, RdRp, Spike A, Spike B) by means of RT PCR. The negatives in the RT PCR column show that none of the samples came out positive on a standard PCR assay. Indeed, the authors say that all the positives only emerged after two rounds of amplification, i.e., using the product of a first PCR as input into a second PCR: “All samples that we identified as SARS-CoV-2-positive (pre-pandemic and pandemic cases) were positive only after two rounds of amplification.”
What this means is we may be talking about tiny amounts of RNA in the samples being picked out and amplified, significantly increasing the chances of false positives due to contamination or cross-reaction with other viruses. Two rounds of PCR are never normally needed to detect SARS-CoV-2 in infected individuals. The researchers say the weak results are a result of “low viral load” – though if so, it would seem to make the premise of their study, that these people were in hospital with measles symptoms as a result of Covid infection, somewhat implausible. How can a virus at an infinitesimally low concentration cause a measles rash?
Be that as it may, what caught my eye is that all the pre-pandemic positives in the table are positive for just one fragment of the virus RNA, except for one. That one sample (number nine) was taken from a woman of 25 years of age in southeast Milan on December 15th 2019. It was positive for three fragments, making it the strongest evidence in the study of the presence of a true infection.
This date – December 15th – rang a bell because I recalled that it coincided with the earliest PCR positive from wastewater studies in Italy, which identified SARS-CoV-2 RNA in sewage, once again from Milan, on December 18th 2019 (see table and charts below).
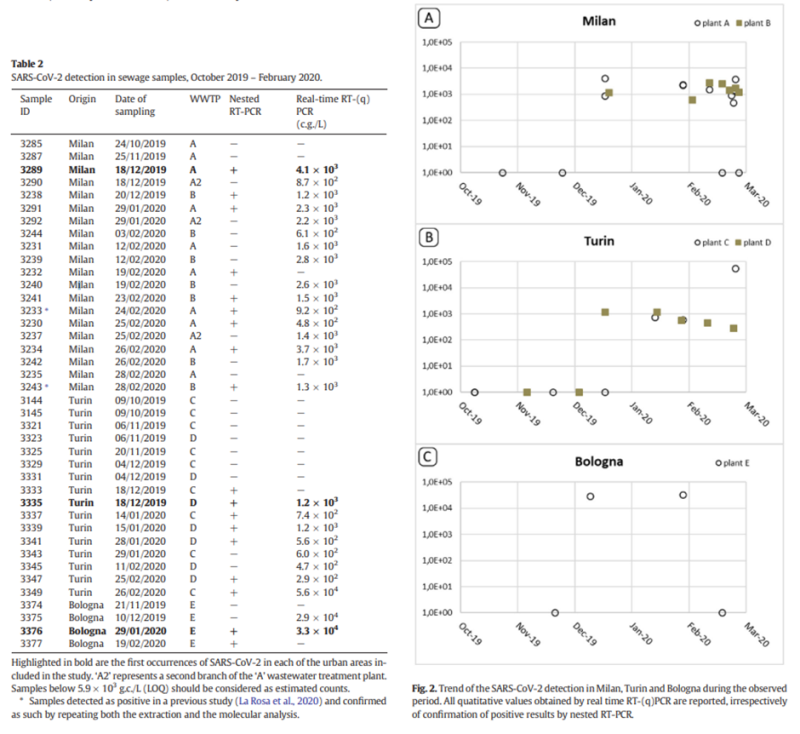
This coinciding of the first Italian wastewater positive with what may be the first or only real positive from the Amendola study struck me as a pointer that shouldn’t be ignored. It suggests that the appearance of the virus in Italy may have been more like November than September 2019, and that Amendola’s earlier weak results were more likely to be false positives. Note that other wastewater evidence seems to confirm this picture, for instance the Brazilian sewage that went PCR-positive from November 27th 2019 but not earlier.
It seems most unlikely that the virus could have been present in a quarter of people in Lombardy hospitals with measles symptoms in September, October and November 2019 but fail to show up in wastewater until mid-December. Wastewater is a lagging indicator, sure, but 25% is a huge proportion and it doesn’t lag that much.
If my interpretation is correct and the virus was not spreading internationally before October 2019, how can we explain the antibodies (IgM, IgA, IgG) that were present in many of the study’s early samples, and also in 12 of the control samples dating back to October 2018 (and possibly earlier, had they been tested)? Amendola and colleagues themselves do not suggest that the ‘SARS-CoV-2’ antibodies they found in the control samples (from October 2018 to July 2019) were genuinely from the virus, so by implication deemed them to be cross-reacting.
This question gains force when we realise that Amendola et al. were not the only ones to find such early antibodies. Apolone and colleagues also found Covid-19 antibodies in stored Italian samples (this time from lung cancer screening) dating back to September 2019 (regrettably in this case the researchers did not test any samples earlier than this or undertake any testing for viral RNA).
The most obvious explanation for these early antibodies would be cross-reaction with similar antibodies. However, what this explanation doesn’t seem to account for is the striking fact that the early antibodies in both the Apolone and Amendola studies were concentrated in the parts of Italy and Lombardy that, come February and March 2020, were the worst hit by the virus. This correspondence is a striking coincidence. It needs some kind of explanation. But what?
Here is the geographical distribution figure from Apolone. The clustering of pre-pandemic antibody positives can clearly be seen in Lombardy and, within Lombardy, in Bergamo, the places worst affected in spring 2020. In fact, over half of Apolone’s pre-pandemic antibody positives were in Lombardy.
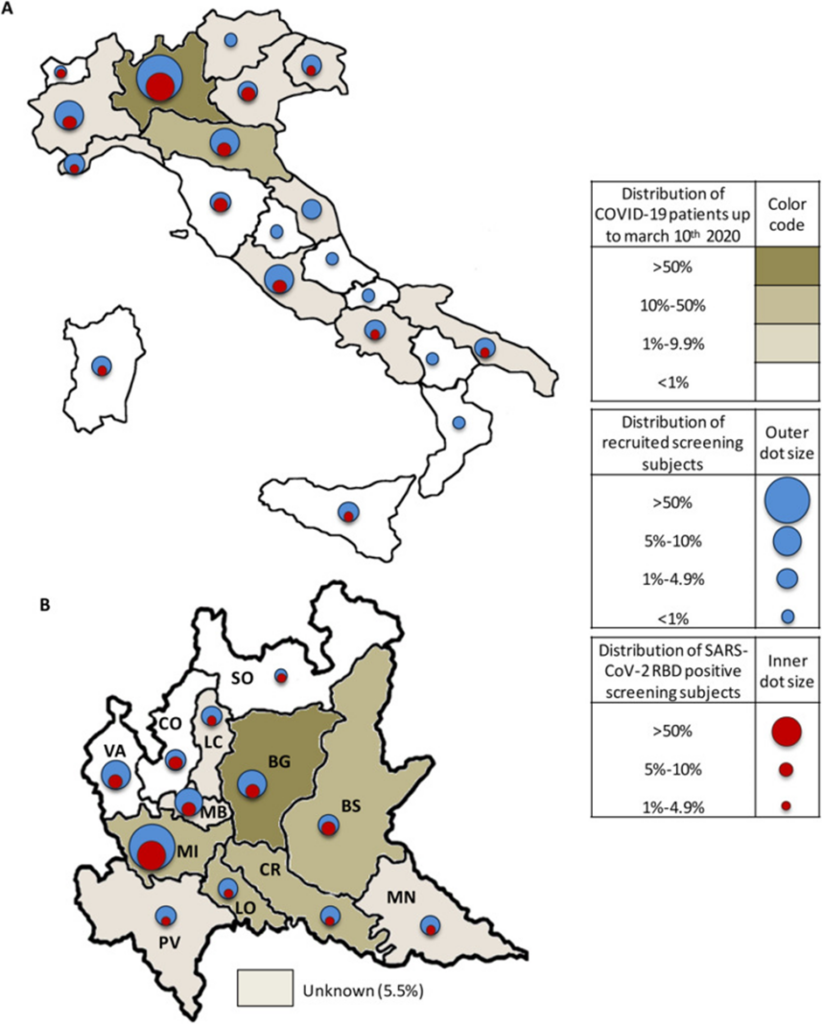
Amendola shows a similar pattern, reporting its early positives in the parts of Lombardy that were later the worst affected in spring 2020.
The first pre-pandemic cases were mainly localised east of Milan and Brescia (September-October 2019), while later cases were identified in northwestern Milan (November-December 2019). No cases were reported from Como, Monza-Brianza and Varese, cities not particularly affected by Covid-19 during the first epidemic wave
If, as I have suggested, these antibodies were not from Covid-19 (because the virus didn’t arrive in Italy until around November 2019) but were due to cross-reaction with similar antibodies, how do we explain them being concentrated in exactly the places that later suffered strong first Covid waves?
This is a question that I suggest those studying Covid origins and early spread look into properly. Is it just because those areas are particularly susceptible to coronavirus infections? Perhaps, though if so it would be interesting to know why.
For my part, I wonder if it may be a result of antibody-dependent enhancement (ADE). This is a phenomenon where, to quote Wikipedia, the “binding of a virus to suboptimal antibodies enhances its entry into host cells, followed by its replication”.
Could these similar, cross-reacting antibodies explain why Lombardy was so badly affected in the first wave – did the population suffer from a very unfortunate case of ADE that greatly worsened the spread and progress of the disease in the first wave? Might this have been the case in other early hotspots too, such as New York? We tend to think of cross-reacting antibodies as providing additional protection and possibly explaining why some people and regions had a milder course. But could they, where ADE occurs, also explain the opposite?
It’s certainly worth considering as we continue to look into when exactly this virus first appeared and where it came from.
Republished from The Daily Sceptic
Disclaimer
Some of the posts we share are controversial and we do not necessarily agree with them in the whole extend. Sometimes we agree with the content or part of it but we do not agree with the narration or language. Nevertheless we find them somehow interesting, valuable and/or informative or we share them, because we strongly believe in freedom of speech, free press and journalism. We strongly encourage you to have a critical approach to all the content, do your own research and analysis to build your own opinion.
We would be glad to have your feedback.
Source: Brownstone Institute Read the original article here: https://brownstone.org/

